| Artiklen er skrevet på baggrund af en veterinær specialeopgave skrevet af Sanja Kyhse-Andersen og Julie Burup Jakobsen med Lise Nikolic Nielsen og Rebecca Langhorn som vejledere. |
Introduction
Recognition and management of acid-base disorders have become an increasingly common part of the clinical workup in many private veterinary practices, as imbalances in acid-base homeostasis are commonly identified in critically ill patients1,2.
The acid-base status of the body is controlled by the concentration of H+, measured as pH, its negative logarithm3. Serious deviations from normal pH (approximately 7.4) will affect cellular metabolism and thereby organ function4. Acid-base disturbances are caused by an accumulation of either acid (acidosis) or alkali (alkalosis) in the body3. The disturbances can be further divided into being of either metabolic or respiratory origin.
All critically ill animals should be examined for acid-base disturbances. Additionally, for animals presenting with gastrointestinal, renal, endocrine, cardiac, respiratory, and neurological diseases, acid-base evaluation might contribute additional information to a patient’s workup2. Characterization of disturbances may provide insight into underlying pathophysiology, direct diagnostic resources and monitor response to treatment.
A patien's acid-base status can be evaluated through a blood gas analysis5. The blood sample should always be collected from the jugular vein into an anticoagulated syringe. After blood collection, the sample should be stored anaerobically and analyzed by an automated analyzer within 30 minutes of collection.
The blood gas parameters relevant for acid-base analysis include pH, Pco2 and HCO3- 3. The first step is to simply differentiate between acidemia and alkalemia. A lower pH corresponds to acidemia and a higher pH to alkalemia.
In case of either acidemia or alkalemia, physiological buffers (CO2 and HCO3- ) can help analyze the pH in the body. Compensation through these buffer systems occurs by the body adapting either its ventilation or renal function6. The lungs help maintain a normal pH by adjusting the ventilation rate and thereby the concentration of CO2. A respiratory compensatory response is initiated within minutes and optimized after 12 to 24 hours and is therefore responsible for the immediate stabilization of the blood pH7. The kidneys are responsible for the slower fine-tuned metabolic compensatory regulation which consists of H+ excretion and HCO3- excretion, reabsorption, or production7,8 (Figure 1). The renal system responds within hours but requires 2-5 days to achieve maximum effect3.
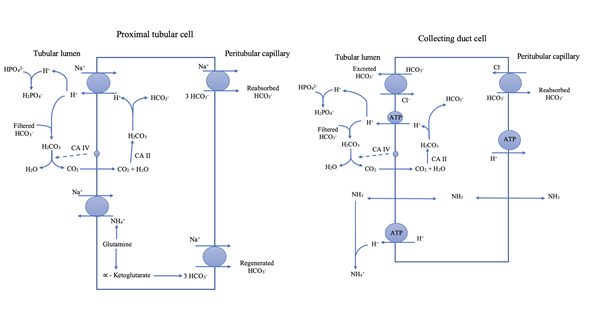
Figure 1. H+ excretion occurs in the proximal tubules by a Na+-H+-exchanger and an electrogenic H+-ATPase pump in the collecting ducts -HCO3 - is reabsorbed from the tubular fluids to the peritubular capillary via a Na+- HCO3 - -cotransporter in the proximal tubulus3. HCO3 - is regenerated by titration of phosphate and by ammonium excretion7,8. The collecting ducts are capable of HCO3 - excretion in response to alkalemia by a HCO3 - -Cl--exchanger into the luminal fluid and reabsorb H+ into the peritubular capillary via a H+-ATPase pump8
When evaluating an acid-base disturbance, the parameters Pco2 and HCO3- are
measured both for determination of the origin of the disturbance (respiratory versus metabolic) and for evaluation of the compensatory response3. In case of a primary metabolic disturbance, the compensatory response ought to be mainly respiratory and vice versa. If an acid base disorder is simple (meaning solitary, with only one cause), Pco2 and HCO3- should always deviate in the same direction because they must be balanced in the body. If that is the case, interpretation is also simple. If pH and Pco2 deviate in the same direction, the primary condition is metabolic. If they deviate in opposite direction, the primary condition is respiratory. In theory, the same interpretation could be accomplished applying HCO3- in place of Pco2, but due to renal compensation being slower, it is highly recommended to use Pco2 for this purpose.
Both dogs and cats compensate metabolically to respiratory acid-base disorders. Humans and dogs are also able to develop respiratory compensation for a metabolic disorder (a low Pco2 will compensate for a metabolic acidosis, and a high Pco2 will compensate for a metabolic alkalosis)9–11.
If insufficient respiratory compensation occurs in a dog, it would be clinically relevant to investigate whether a concurrent clinical abnormality exists in the respiratory system, leading to a so-called mixed acid-base disorder (two or more simultaneous acid-base disturbances)3. While a similar strategy might be considered in cats, the few available studies on metabolic acidosis in cats show either a minimally decreased or a normal to high Pco2, indicating an insufficient or lacking respiratory compensation in this species12–15. None of the studies investigate this area of concern any further, and an underlying physiological cause for the difference between species has not been established. The scarcity of studies in the field leads to the objective of this study: To evaluate the respiratory compensation in a population of cats with metabolic acid-base disturbances.
Materials and methods
This study was a cross-sectional study, evaluating blood gas analyses from cats examined at the University Hospital for Companion Animals from 2003 to 2018.
Patients included were cats for which the responsible veterinarian considered a blood gas analysis relevant. Only blood gas analyses performed on a GEM Premier 3000 blood gas analyser were included. Cats with normal acid-base balance, with a primary respiratory acid-base disorder, or with mixed disorders were excluded. Cats with a primary metabolic acid-base disturbance and with concurrent confirmed or strongly suspected respiratory disease were also excluded in order to avoid bias when interpreting Pco2.
Data collection
Data was collected retrospectively from results of all blood gas analyses performed on cats from 2003 to October 2018. These results were obtained via medical records from VetNetManagement, Vetvision and from the GEM Premier 3000 blood gas analyser. From October to December 2018 additional data was collected prospectively by the responsible veterinarian at the University Hospital. The veterinarian performed a general examination of the cat prior to blood collection, and informed owner consent was obtained. Blood was collected from the jugular vein with minimal stasis and an amount of 1.5 ml blood was collected into a PICO50-Aspirator syringe. Blood gas analysis was completed within 20 minutes of blood collection.
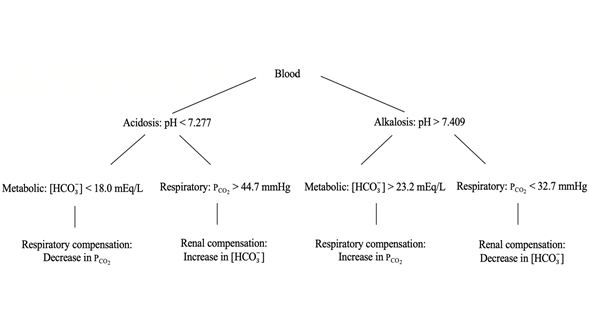
Figure 2. An overview of the four primary acid-base disturbances. The first step in determining the acid-base status of a patient is to differentiate between acidemia and alkalemia based on pH. The next step is to establish whether the acid-base disturbance is of respiratory or metabolic origin. This can be determined by evaluating either Pco2 or HCO3 - .
Included data
For each cat, the following clinical and clinicopathological findings were included: signalment, history (especially regarding recent vomiting/diarrhea), clinical exam, complete blood count (hemoglobin, and hematocrit), biochemistry (Na+, K+, Cl-, Ca2+, albumin and creatinine), and urinalysis (pH, ketones, and glucose). Only blood and urine samples analysed within 24 hours of the corresponding blood gas analysis were included.
Data processing
Acid-base status for each cat was classified as shown in figure 2 using the following reference values: pH (7.277 – 7.409), Pco2 (32.7 – 44.7 mmHg) and HCO3- (18.0 – 23.2 mEq/L)16.
In this study, HCO3- was chosen to classify all acid-base disorders as of either metabolic or respiratory origin, since the compensatory reaction in Pco2 to a metabolic acid-base disorder is uncertain in cats. The midpoint reference of HCO3- (20.6 mEq/L) was used to determine HCO3- direction.
Calculation of compensation
After identifying the primary metabolic acid-base disturbance, the expected respiratory compensation was calculated using the following guidelines for expected compensation:
- Metabolic acidosis: Pco2 should decrease by 0.7 mmHg for every 1 mEq/ml decrease of HCO3-.
- Metabolic alkalosis: Pco2 should increase by 0.7 mmHg for every 1 mEq/ml increase of HCO3-.
The values are calculated mean values from Morris and Leisewitz17 which are derived from a number of studies performed on dogs and very few cats. Due to the limited number of cats, our calculations for respiratory compensation had to be based on values derived from dogs.
To calculate the respiratory compensation, the following equation was applied18:
| PCO2 expected = Midpoint PCO2 - ΔPCO2 |
ΔPCO2 = Δ[HCO3-] x 0.7 Δ[HCO3-] = Midpoint [HCO3-] - Patient [HCO3-]
The expected Pco2 was compared to the patient’s measured Pco2 using this equation:
| PCO2 difference = PCO2 patient - PCO2 expected |
The present study defined satisfactory compensation as no significant difference between calculated and expected Pco2.
Statistical analysis
The distribution of our data was evaluated by a D’Agostino & Pearson normality test. The difference between the patients measured and expected Pco2 was assessed by a one-sample, two-tailed t-test. Two t-tests were calculated: One for metabolic acidosis and one for metabolic alkalosis. Statistical significance was defined as P<0.05. All graphs and statistical tests were performed in GraphPad Prism version 8 (GraphPad Software, San Diego, California, USA).
Results
A total of 282 cats were included in the study. Of these, 42 were excluded due to missing values (pH, Pco2 or HCO3- ), and 61 were excluded because their blood gas analyses were not conducted on the GEM Premier 3000. The acid-base disorders for the remaining 179 cats were classified as demonstrated in Figure 3.

Figure 3. The distribution of acid-base disturbances of the 179 cats included in this study.
The 66 cats with metabolic acid-base disturbances were included for further processing. Of these, five cats were excluded due to a concomitant confirmed or suspected respiratory disease as such was likely to affect the respiratory compensation. The study exclusion process is illustrated in figure 4.
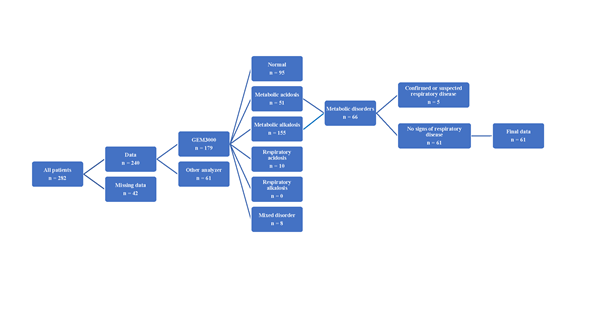
Figure 4. The sorting process of all 282 cats that have had a blood gas analysis performed during the last 15 years at the University Hospital for Companion Animals at the University of Copenhagen, resulting in 61 cats included in the final population.
Of the 61 cats included in the final population, 37 were male (3 of 37 neutered) and 24 were female (4 of 24 neutered). The cats’ age ranged from 7.8 months to 16.9 years with a mean age of 6.5 years. The diagnoses of the 61 cats are listed in Table 1.
No significant correlation was found between the Pco2difference and the hematological and biochemical parameters known to influence acid-base homeostasis (hematocrit, hemoglobin, Na+, K+, Cl-, Ca2+, albumin and creatinine), and no significant findings were identified for the urine parameters (pH, ketones, and glucose).
|
Metabolic acidosis (n=48) |
|||
| Urogenital system (n = 24) | Endocrine system (n = 5) | Other (n = 15) | Multiple diseases (n = 4) |
| Obstructive FLUTD (n = 6) |
Diabetic ketoacidosis |
Hypertrophic cardiomyopathy (n = 1) | Diabetes mellitus + renal problem (n = 1) |
| Renal failure (n = 8) |
Retained placenta (n = 1) | Gastrointestinal problem + renal problem (n = 1) |
|
| Other renal (n = 10) |
Intoxication (n = 2) |
Pancreatitis + renal failure + neurologic problem (n = 1) | |
| Shock (n = 2) | Pancreatitis + gastrointestinal problem (n = 1) | ||
| Neurologic (n = 3) | |||
| Gastrointestinal (n = 3) | |||
| No diagnosis (n = 3) | |||
| Metabolic alkalosis (n = 13) | |||
| Gastrointestinal system (n = 4) | Neurologic system (n = 4) | Other (n = 4) | Multiple diseases (n = 1) |
| Foreign body (n = 2) | Intoxication (n = 2) | Urogenital disease (n = 2) |
Constipation + renal problem |
| Other (n = 2) | Epilepsy (n = 1) | Pancreatitis (n = 1) | |
| Other (n = 1) | No diagnosis (n = 1) |
||
Table 1. Diagnoses of the included cats. This table shows the distribution of diagnosis amongst the 61 included patients in this study with respectively metabolic acidosis and metabolic alkalosis.
A statistically significant difference was found between measured and expected Pco2. The mean for metabolic acidosis was 3.291 mmHg (p <0.0001), and the mean for metabolic alkalosis was -3.197 mmHg (p=0.0006), indicating that the examined population of cats was not able to perform satisfactory respiratory compensation.
Discussion
It is important to determine the respiratory compensation to metabolic acid-base disturbances in cats in order to optimise choices of further diagnostics and treatments. Our results demonstrate a significant difference between the expected and measured Pco2 in the examined population, indicating that cats with metabolic acid-base disorders do not compensate when compared with what is described as normal for dogs and humans9–11.
This finding was first described in a case report in 1986. A cat with severe acidemia demonstrated low plasma bicarbonate concentration and normal venous Pco2, indicating a metabolic acidosis without respiratory compensation13. Subsequently three additional studies have been performed, failing to detect respiratory compensation in acidemic cats with chronic renal failure (n=13) and in a group of cats with metabolic acidosis of different causes (n=11)14,19. Finally, one study, in which 6 cats developed metabolic acidosis, reported a small decrease in Pco2 demonstrating a detectable, but insufficient respiratory compensation15.
A study from 1986, with eight cats fed ammonium chloride as a urine acidifier, showed a decrease in blood Pco2 and HCO3- without a decrease in blood pH20. This indicated that cats might be able to perform minimal respiratory compensation to prevent acidemia in case of small alterations in the acid-base status of the body. However, our study showed a lack of respiratory compensation for cats in which metabolic acidosis had already developed.
Importantly, the formula used to calculate the respiratory compensation is based on a constant (0.7 mmHg/mEq) derived from studies in dogs, due to the limited evidence of acid-base interpretation in cats. Using the canine constant was considered appropriate because the study focus was to evaluate whether blood gas analysis in cats can be interpreted based on the canine and human criteria. While this approach confirmed that cats do not compensate to the degree observed in canine metabolic acid-base disturbances, it was less optimal than having specific feline criteria. With inspiration from a study by Penman, Luke, and Jarboe21, an attempt of creating our own feline constant was then carried out by plotting Pco2 and HCO3- in a linear regression. For acidosis, the resulting constant was 1.16 and for alkalosis it was 0.77. These values are very similar to values in human studies, which are 1.2 for acidosis and 0.7 for alkalosis22. However, a limitation for this calculation is that it was not possible to compare our Pco2 and HCO3- concentrations to their respective baseline values.
Our results influence the diagnostic process of the veterinarian. When a metabolic acid-base disorder is evident with no compensatory response in Pco2, the veterinarian might suspect an underlying disorder affecting the respiratory system. For a cat, based on these results, this could lead to time-consuming and expensive diagnostic measures searching for a non-existent respiratory disease.
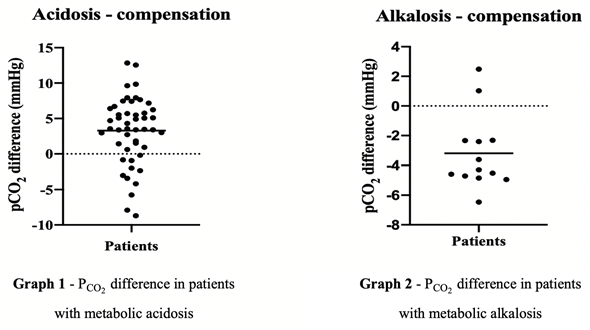
Figure 5. The difference between the measured and the expected Pco2 for the 61 included cats with respectively metabolic acidosis and metabolic alkalosis
In the present study, the most common acid-base disturbance in cats was metabolic acidosis (51 of 179 – 28.5%) followed by metabolic alkalosis (15 of 179 – 8.38%). This is not consistent with the results of Hopper, and Epstein23 who found the most common acid-base disturbance in cats to be a mixed disorder with a metabolic acidosis combined with a respiratory acidosis.
The most common cause of metabolic acidosis in this study was renal disease which is coherent with Hopper, and Epstein23. This is supported by a study from 2003 in which 22% of 59 cats with chronic kidney disease were diagnosed with metabolic acidosis14. The most frequent cause of metabolic alkalosis in this study was not possible to identify due to the small number of cats with this abnormality, and since the distribution of diseases was widespread.
No correlation between Pco2 difference and examined hematological and biochemical parameters was found. These results demonstrate no relation between the selected blood parameters and respiratory compensation, indicating that further investigation into this area is likely not necessary.
The present study has some limitations, mainly inherent to retrospective data collection for which specific clinical information is sometimes difficult to obtain. Hence many cats were excluded. Also, the constant (0.7 mmHg/mEq) applied in our equations was based on canine values. If a specific feline constant was established, it would optimise the interpretation of the feline acid-base analysis in clinical practice. However, because we applied a canine constant to feline data, we were able to confirm that cats do not mimic dogs in their respiratory compensation as seen in a blood gas analysis.
Conclusion
Our primary purpose with this study was to establish, with greater certainty, whether respiratory compensation in cats is different from that described in dogs, as indicated by a few previous studies. The population examined in this study was in agreement with previous research, and the study found insufficient respiratory compensation in cats with metabolic disorders based on current companion animal guidelines drawing mainly on canine studies. This finding is of relevance in clinical practice because the lack of compensation seen on blood gas analysis appears to be of little consequence to the cat, unlike dogs for which one should rule out a respiratory disorder in these situations.
Further research into the underlying physiology in cats is considered important in order to explain alternative physiological pathways of compensation in cats and to develop specific guidelines for feline acid-base interpretation.
Acknowledgements
We would like to thank the veterinarians and veterinary technicians involved in performing blood gas analyses on the included cats and the owners of the cats for allowing them to be included in the project.








