Introduction
Biotherapy is defined as the use of living organisms, or derivatives thereof, as a treatment for a disease. These living organisms may occur naturally in the body or may be produced in the laboratory. In the horse, one of the most common living organisms used as a biotherapy are mesenchymal stem cells (MSC). Their use is currently, and almost solely, directed as a treatment for osteoarthritis (OA), and/or tendonitis (TEN), particularly of the superficial digital flexor tendon (SDFT). Both OA and TEN are highly frequent in the equine athlete, triggered and maintained by a complex interaction between exercise and/or repetitive micro trauma [1]. Both diseases lead to significant economic repercussions in the equine industry, particularly due to treatment costs, lower performance and a shorter athletic career.
OA is the most prevalent cause of reduced performance in the horse [2]. It is characterized by synovitis, progressive cartilage degeneration, subchondral bone remodeling, osteophyte formation, and in the long-term loss of function (lameness) [3]. During OA, inflammatory cytokines are produced, particularly interleukin-1b (IL-1β), tumor necrosis factor-α (TNFα), and matrix metalloproteinases. These cytokines disrupt the anabolic/catabolic balance of growth factors such as insulin-like growth factor-1 (ILF-1), transforming growth factor beta (TGF-β), and basic fibroblast growth factor, amongst many others, provoking a chronic and degenerative process within the joint and its adnexa (cartilage, subchondral bone, joint capsule) [3,4].
Medical treatment of OA has largely consisted of intraarticular (IA) injection of corticosteroids and systemic treatment with non-steroidal anti-inflammatory drugs (NSAID), combined with rest, corrective farriery and progressive exercise remission. The main goals being to reduce pain and lameness, and to slow disease progression. Different corticosteroid molecules have been suggested for low (i.e. distal intertarsal joint) or high (i.e. fetlock) motion joints [5]. Corticosteroids impede degradative responses in cartilage during the inflammatory process, but detrimental effects on the articular cartilage have also been demonstrated [6]. Furthermore, when injecting a corticosteroid
into low-motion joints, where further degradation could be resolved by fusion (i.e. distal intertarsal joint), deleterious effects on other more mobile joints could be expected [7]. Corticosteroid use hence may seem beneficial at early inflammatory periods of arthritis, by diminishing pain and allowing the horse to get back in training sooner. However, the effect is only short-lasting and in the long term, there has been established a positive association between their use, and subsequent higher musculoskeletal injury rates [8].
TEN is one of the most common musculoskeletal injuries sustained by horses competing in all disciplines, with a particularly high prevalence reported in show jumpers and in racing Thoroughbreds [9]. The forelimb (SDFT) is far more likely to be injured than any other tendinous structures in the equine limb, with ∼90 % of tendon injuries occurring to this structure, and most are localized in the metacarpal region. Once TEN manifests clinically, the disease has been through a degenerative phase already, which does not have any clinically signs, and more importantly, no reparative response, progressively destroying the tendon until clinical signs appear [10]. Commonly, overstrain injuries are described as core lesions, which represent breakage of fiber bundles within the tendon structure, with resulting areas of blood and inflammatory fluid collection. These will appear as a well-defined hypoechogenic area during ultrasound examination. The affected area will contain hematomas and inflammatory infiltrates, which will result in lower echogenicity. During the tendon healing process, several growth factors are involved, such as TGF-β and basic fibroblast growth factor. The affected tendon region will form a fibrotic scar, giving rise to a thicker and harder tendon, with increased stiffness. In consequence, the tendon will be less elastic, with a persistent deficient structural architecture, which will make it prone to recurrent lesions [11–13].
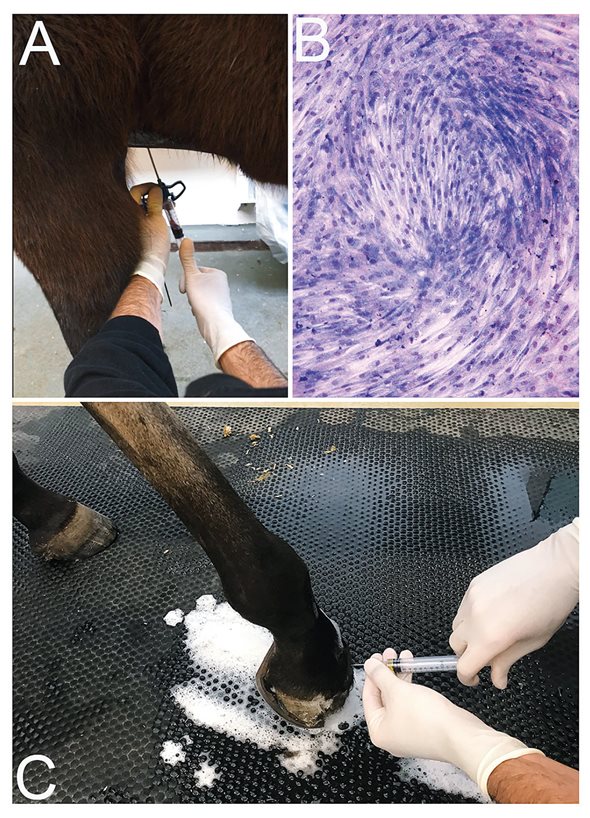
Figure 1. MSC therapy. Bone marrow aspirate is done under aseptic conditions (A), and after cell amplification of about two weeks; MSC can be identified by its fusiform like shape (B), giemsa staining. The final product is prepared for intraarticular injection under sterile conditions (C).
In the acute stage, treatment of TEN should focus on reducing inflammation to avoid further tendon degeneration. This can initially be achieved by cooling the limb combined with provision of extra support (bandaging). When assessing the management of the tendon injury itself, many treatments have been proposed, including corrective shoeing, compression, coaptation and intralesional medication, although few have convincing supporting evidence of efficacy [14]. The basic idea to keep in mind, when treating an affected tendon or ligament, is how to avoid recurrence of the injury, and hence how to produce a more tendon-like repair rich in collagen I, with less fibrotic tissue (< collagen III), and in this matter, maintaining the functionality and elasticity of the structure. Intralesional therapy has focused on these objectives. Polysulfated glycosaminoglycans (PSGAGs) and hyaluronic acid (HA) have been suggested as possible therapies [14]. PSGAGs inhibit the catabolic metabolism but have no effect on diminishing fibroblast synthesis. Studies using HA have shown no significant advantages when comparing reinjury rates, and PSGAGs showed an improvement in horses returning to competition, but the recurrence rate was not measured [15,16].
Biotherapies have been suggested for orthopedic injuries in horses since the early 2000s [17], and currently a plethora of various products are or have been available. The use of mesenchymal stem cells (MSC) is among the most commonly reported. These cells have shown promise in the treatment of inflammatory musculoskeletal conditions including OA and TEN [18].
Mesenchymal stem cells as biotherapy
MSC are multipotent adult stem cells, which can be found in many tissues such as umbilical cord, bone marrow (BM) and fat. MSC can proliferate and differentiate into different tissues including bone, cartilage, muscle and fat [14]. The rising interest in MSC is due to its properties on multipotency, proliferation, clonogenic potential, paracrine action, and immunomodulation activity. MSC can be isolated from different tissue sources, most often from (BM), peripheral blood or adipose tissue (AT). Selection of source will basically depend on ease of access of harvesting, and the need for local or general anesthesia, as well laboratory facilities.
The most accessible approach to obtain a bone marrow aspirate is from the sternum, which can be done in the standing horse. The method consists of sedating the horse, aseptic preparation of the region combined with infiltration of local anesthesia. After identification of the puncture site (which can be facilitated with ultrasonography) a trocar (Jamshidi needle) is inserted into a sternebrae, and the bone marrow is collected by aspiration [19]. The sample is then further manipulated under laboratory conditions where MSC will be isolated and cultured until the necessary number of cells is obtained (Figure 1).
Another common approach is the proliferation of MSC from an AT sample. For this, it is often recommended to collect the fat from the gluteal region. This can also be performed with the animal standing. To obtain the necessary amount of fat tissue, a 10 to 15 cm long incision is generally necessary. The incision is made through the skin, parallel to, and about 15 cm lateral to the vertebral column [20], and with the use of surgical equipment, the AT tissue is harvested over the superficial gluteal fascia. The sample will need further manipulation in the laboratory, which is more elaborate than with the bone marrow aspirate, but the objective is the same, meaning the isolation and amplification of MSC.
Liposuction approaches have been reported in humans, probably being less invasive than seeking a bone marrow aspirate and avoiding a large skin incision. However, due to the minced adipose tissue obtained during the harvesting procedure, a larger harvesting surface is necessary [21]. The need of a more specialized equipment (liposuction) has probably limited this technique in the horse, although the large harvesting incision could potentially be avoided with this approach.
Even if both sources will result in MSC isolation, cells are not intrinsically the same. The yield [22] and proliferation rate [23] are much higher from AT than from BM sources, but the need of a relatively large incision and the possible complications (dehiscence, seroma, infection) to obtain AT, quickly undermines these advantages. Interestingly, chondrogenic [20] and osteogeneic [23] capacities were stronger in BM cells when comparted to AT origin, which could make them more advantageous when aiming for OA therapy [24]. Once under culture conditions, phenotype characterization can be performed to ensure the quality of the amplification protocol.
As reported elsewhere [25], MSC will adhere to the plastic surface of the culture box, they will have proliferative capacities, and will show a fibroblast fusiform-like shape. This is important to consider during the amplification process, as a heterogeneous cell population, with different stages of maturity and lineage commitment will compose the initial cell isolate [26]. Further characterization can be done with cell surface markers and gene expression patterns, before and after induction to specific tissue lineages, if the objective is to differentiate the cultured cells into a specific lineage (specific type of cells), such as cartilage precursor cells.
MSC can be used as either an autologous source, meaning that cells are harvested from the same individual where they will later be injected, or they can be used as allogenic cells, which means that the cells are harvested from one individual and then injected into different subjects of the same species. Scarce reports exist in the horse that compare the advantages or disadvantages of autologous versus allogenic cell products.
The quick availability of MSCs has been advocated as an advantage of allogenic cells that can be kept frozen and ready for immediate use, as with any other type of medication. However, in acute cases, it is relevant to wait for the affected tissue to be less inflamed, before initiating cell therapy, as the initial ongoing inflammatory reaction will reduce implanted cell viability. This inflammatory phase will last about two weeks [27,28], which is then the average time needed to amplify autologous cells to reach a sufficient number for implantation. In the case of more chronic cases, the need for immediate therapy becomes less crucial, where immediate implantation has then insufficient justification.
Another question which has been raised, is the different safety of allogenic versus autologous cells. Specifically, attention has been directed towards avoiding adverse articular reactions such as flares, due to immune mediated responses when implanting allogenic material particularly if repeated injections become necessary [29].
The articular environment is considered to be prone to immune reactions. The synovial membrane is a confined layer that provides a blood to joint barrier, and the presence of a specific articular macrophage-like synoviocyte is thought to lead to enhanced immune responses and antigen recognition when compared to other tissues [30]. Even with this particular immune predisposition, in a systematic review on the safety of MSC therapy in humans, on 844 procedures with a mean follow-up of 21 months, there were no adverse effects noted [31] and allogeneic MSC use was well-accepted [29]. On the other hand, one of the few safety studies in horses, comparing both allogenic and autologous sources, with single or repeated articular injections, concluded that the repeated injection of allogeneic MSCs provoked an increased number of adverse joint reactions [29]. Importantly, this study showed that intraarticular injection of autologous MSCs did not result in any adverse responses such as higher cell count, joint effusion or pain.
In contrast, allogenic cells caused lameness and a significant increase in cell counts, particularly when reinjecting the product four weeks later, which means that immune responses to allogeneic MSCs could negatively impact cellular longevity and efficacy [32]. In another study, where arthritis was induced before cell implantation, results showed no differences between allogeneic or autologous MSC [33], however, it was not stated if the ongoing induced strong inflammatory joint process would have masked the adverse effects of allogenic cells.
With contradictory results, an autologous source would be advantageous if laboratory facilities are available, as cells will not have to be frozen and unfrozen, and then transported. Another consideration is that the majority of the implanted cells die relatively quick, with around only 24 % of them being retained at the site of implantation [34]. With this, one must consider factors that influence this poor cell retention, such as reduced cell viability after thawing [35], transport where cell viability can decrease by 70 % [36], and damage during the process of injection, where significant increase (>9 %) in dead cells was found when injected through a 21G or 23G needles, in contrast of using wider gauge (18G). This can lead to two adverse consequences: First, a reduced efficacy, as the planned viable number of cells to be implanted end up being significantly lower, and secondly the presence of dead cells, which may induce inflammation and hence potentiate the afore mentioned unwanted joint reactions. In addition, increasing the number of implanted cells to increase the number of viable cells, will eventually also increase the amount of dead cells [37].
The initial theory was that MSC will survive and differentiate, once implanted in the affected tissue, and then help regenerate the damaged tissue. However, we know now that the function of MSC is more complex than that and with a multitude of potential actions [33]. Consequently, more recent explanations of the positive effects seen after MSC implantation is their marked anti-inflammatory and immuno-modulatory effects, facilitating a healthier healing, rather than a tissue regeneration per se, although much is still unknown about the specific mode of action of MSC.
Tendonitis biotherapy with MSC
The beneficial effects of autologous MSC use on TEN in equine patients, particularly in core lesions of the SDFT or suspensory ligament, have been investigated since the early 2000s. Because of the poor functionality of scar tissue formed by natural healing, treatments should aim, ideally, at regenerating a tissue with the same elastic and tensile properties as close to normal tendon as possible. Hence limiting the loss of function and reducing the recurrence rate [38].
It has been shown that BM-MSC therapy will increase perfusion and neovascularization of healing tendon lesions [39], improve the deposition of certain extracellular matrix proteins such as type I collagen and cartilage oligomatrix protein (COMP), and reduce the levels of collagen type III (indicative of fibrotic scar tissue) [40].
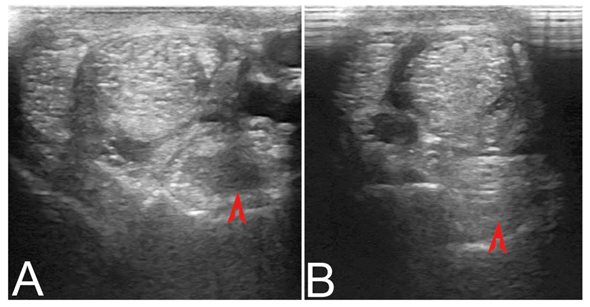
Figure 2. Lesion treatment in a suspensory ligament. Ultrasound examination of the suspensory ligament on a hind limb, can be diagnostic for the presence of a lesion (A). Injection of MSC is done ultrasound guided to assure that cell implantation is done within the lesion. A routine control ultrasound is done six weeks later, where it can be stated if the treated structure is healing properly (B).
Furthermore, implanted cells will line up along the collagen fascicles, and cells can survive within the tendon for up to 4 months after implantation, although in relatively low numbers [41]. What exactly happen to those cells that survive after implantation is not fully understood. They may either differentiate into tenocytes, synthesize tendon matrix, or they may act in a paracrine or trophic fashion to stimulate new tissue production by the local cells. In addition, MSCs are thought to have a profound anti-inflammatory effect via their inhibition of T cell mediated responses [42]. MSC have been implanted into surgically created tendon defects in multiple experiments using laboratory animal models, with almost universally positive outcomes.
Overall, data shows that therapy with BM-MSC is associated with better histological results in naturally occurring TEN than in experimentally induced injuries, probably due to differences in etiopathogenesis [39]. Furthermore, their implantation produced the most rapid ultrasonographic improvement at 6 weeks post therapy (Figure 2), suggesting an enhanced reparative process possibly preventing additional tendon degeneration [43].
In practice, naturally occurring tendon lesions in racehorses treated with MSC, resulted in a significant lower reinjury rate, averaging 18 to 27 %. MSC treated tendon lesions showed improved healing with better histological and immunohistochemical parameters at day 60 and day 150 post treatment, compared to controls, these representing a better longitudinal alignment of collagen and tendon fibers six weeks post implantation, altogether leading to a better functional recovery [40,44–47]. As a result of this applied biotherapy, 79 to 89 % of treated horses came back to work without any complications. The lower reinjury rate (18 to 27 %) corresponds to at least 50 % reduction compared to those horses treated with other approaches [48–50].
Osteoarthritis biotherapy with MSC
The use of MSC for the treatment of OA has had more controversial results, due to the more complex pathophysiology of the disease; different joints, duration and severity, age of the patient, type of activity, the comparison between allogenic versus autologous implantation, treatment dose (number of cells) and type of cells used. The implantation of MSC in the joint is thought to provoke a profound modification of the articular environment, down regulating the inflammatory cascade, consequently the articular environment becomes more suitable for adequate healing [51].
Although still scarce in the literature, equine experimental and clinical studies exist, and even if most of them show beneficial effects, mainly on account of reduction in pain [52], there are overall positive findings. IA injection of autologous MSC in horses resulted in reduced pain and slowed OA progression in a couple of studies using an articular cartilage repair model, and in three other studies using an inflammatory joint model. Even if these studies used different models for joint inflammation, two of them observed a significant reduction in inflammation [53–55]. Furthermore, in an equine meniscal tear model with induced chondral defects, treatment with MSC led to a promising fibrocartilage repair patch 12 months later, whereas control menisci were either partially healed or not at all [56].
More recently, MSC implantation mitigated the effects of joint trauma in a focal cartilage injury equine model [57]. However, since the repaired tissue will be under continued and repetitive stress, it is still imperative to carry cartilage healing studies for longer periods, in order to be able to evaluate the durability of the repaired tissue. Even if MSC use seems to improve the clinical outcome, combination with other types of therapy may be also advantageous, as in an equine model of full thickness lateral trochlear ridge defects, cell implantation together with microfracture resulted in a better cartilage repair (macroscopic, histology, and magnetic resonance) after 8 months, in comparison to microfracture alone [58].
From clinical studies, success rates on horses with OA treated with MSC average 77 %, with 38 % being able to come back to competition at their same level, and 43 % of horses with OA of the femorotibial joint were able to return to work after MSC implantation [20,55]. Human research and clinical use of MSC is in a similar stage, and while studies support the notion that MSC therapy has a positive effect on OA patients, there is also limited high quality evidence and long-term follow up.
A human trial demonstrated that IA injection of MSC into OA knees improved function and reduced pain, and evidence of hyaline-like articular cartilage regeneration was found [59]. A large review on human OA, analyzed 2,662 treated human joints, with a mean follow up of 12 months, and all showed an improvement on pain perception [60], with some studies showing also a decrease of the »poor cartilage index« (where high indexes would indicate degraded cartilage). Even if methodology is inconsistent, analysis of these data suggests an association between MSC therapy and OA symptomatic and radiologic improvement in humans, as it has also been shown in horses in terms of overall clinical improvement, histological scores and inflammatory markers [61].
Discussion
MSC are able to differentiate into several cell types such as osteoblasts, chondrocytes, or adipocytes, have high plasticity, self-renewal capabilities, anti-inflammatory and immuno-modulating actions [14]. Growth factors, cytokines, and microvesicles that are released from MSC may also exert beneficial effects including angiopoietic and antiapoptotic actions [62]. Advantages with autologous applications is avoidance of any potential immune reactions, while the disadvantage would be the lack of immediate availability, if this can be truly justified. However, several studies have shown limited proliferative and differentiative capabilities of cells from middle aged and geriatric horses thus making autologous treatment less relevant in older horses (>18 years old) [24,63]. In contrast, allogenic cells could then eliminate possible age-related deficiencies, and allow immediate treatment with a product with more consistent characteristics, but a constant reliable cell source (animal donors) is essential.
The fact that every MSC donor is different, clearly becomes the most important of the uncontrolled aspects of both allogenic and autologous sources. For example, secretion of important bioactive molecules by MSCs can differ 10-fold between sources [64], and so efficiency could be markedly affected. However, what defines efficiency and/or the potency of MSC? The difficulty arises in the availability of developing a phenotypic predictor in vitro, which would then define the in vivo activity or mode of action. Currently, the functionality of the final MSC product is based upon the isolation and expansion procedures, minimal manipulation, and the injured environment that they are injected into, and consequently their ability to respond to the given environment through transcriptional and translation regulations, followed by the release of specific protein mediators that influence the repair, control of inflammation and infection [62].
One of the main MSC properties that has gained a lot of attention, and that probably explains most of the positive effects found in the literature, is the immuno-modulating capacities of the implanted cells. The immune process often involves suppressing T-cells, activating macrophages, and potentially recruiting neutrophils, and facilitating changes in immune cell activation status [65,66]. MSC lower lymphocyte proliferation, production of tumor necrosis factor-a (TNF-a), and interferon-g (IFN-g), and increase prostaglandin (PGE2) and interleukin-6 (IL-6) secretion [67,68]. These features are believed to largely act therapeutically, as they decrease inflammation, pain, protect tissues from hypoxia/tissue reperfusion injury, and suppress adverse immune responses. Considering then that the pathophysiology of OA is both degenerative and inflammatory, stimulation of local growth by reducing the immune response may be then of beneficial effect on this condition.
This means that MSC therapy, human and equine alike, appears to alleviate the symptoms of OA, potentially halt cartilage damage, and improve the healing quality of damage tendons, at least enhanced enough to reduce reinjury rates by half, thereby allowing these horses a better chance to return to previous levels of competition. Cartilage repair failure may arise because of mechanically inferior cartilage, and its lack of full integration to the adjacent tissue, further impaired by the maintained mechanical stress unavoidable in horses.
The full utility of MSC as a biotherapy is yet not completely understood and hence fully established and widely accepted protocols does not exist. MSC need a solution vehicle when injected, or a scaffold that could improve their implantation, and instead of using sterile saline solution or synthetic materials, cells may benefit more from another biological carriers. A combination of MSC with additional biological products has henceforth been tested with the objective of improving their healing and implantation capabilities. For example, when subchondral bone defects of the medial femoral condyle of horses, were filled with a mix of MSC and autologous fibrin gel, the treated joints had a reduced degree of joint inflammation, and a better aspect of the repaired cartilage compared with the control [69].
Another commonly reported combination is the injection of MSC with platelet rich plasma (PRP), the latter is a blood product, obtained after minimal blood manipulation [70]. The resultant concentration of platelets will give an important release and concentration of growth factors, known for their healing and anti-inflammatory properties. A study combined intraarticular MSC and PRP in equine OA joints. 165 horses were treated and between 78 to 86 % of them improved after 12 to 18 weeks of treatment [18]. The possible synergism found by using different biologicals, cellular and humoral, could then help to achieve further clinical improvement of affected animals.
MSC therapy is a promising therapeutic tool for the treatment of musculoskeletal pathologies in equine practice. The results accumulated so far show evidence that equine patients, affected by naturally occurring diseases, provide more reliable outcomes than laboratory animals. Furthermore, the vast majority of human or equine clinical cases treated with MSC, are constantly reported with a clinical improvement and minimal/rare adverse reactions.
Author’s contributions: GCR and CL developed idea and planned the outline. GCR wrote and corrected manuscript, made and edited figures. CL supervised and revised the manuscript. IMHH assisted manuscript editing and summaries.








