Ovarian cysts are practically non-existent in beef cattle breeding, but in dairy cattle they have an average incidence of 10-12 % (range 3-32 %). 65 % of ovarian cysts resolve spontaneously in the first 40-60 DIM, i.e., from a clinical point of view, only the presence of persistent dominant follicles in the absence of luteal tissue should be considered as truly »pathological«, only if they are over 60 DIM.
This is a problem that must be considered with great care, but at the same time with scientific rigour, regarding diagnosis and treatment.
Figure 1. Cystic degeneration of the ovary is one of the most controversial topics in bovine reproductive management. Today, the classification of ovarian cysts and probably also the therapeutic objective must be reviewed
Introduction
According to the definition coined by Roberts in 1971 (1), an ovarian cyst is an anovulatory structure on the ovary of more than 25 mm in diameter persisting for at least 10 days in the absence of a corpus luteum (CL).
Does this definition still make sense today, in 2021? No!
It is a definition that gives enormous value to the diameter of the anovulatory follicle, whereas today we know that the cyst can be as small as 10 mm in diameter. Furthermore, the presence of an ovarian cyst often coincides with regular cyclicity of the ovary, i.e., with the presence of a corpus luteum, i.e., hormonally unproductive ovarian cysts. The temporality of the cyst, persisting for at least 10 days, has also been questioned, given the possibility that in this time the cyst may be replaced by another cystic structure, which often occurs in the case of small cysts (Anaestro type II).
It is time to review the concept of cystic degeneration of the ovaries.
Vanholder et al. 2006 (2), define ovarian cysts as cystic follicles, i.e., the presence of one or more follicles with a diameter of at least 20 mm, present on one or both ovaries in the absence of active luteal tissue.
However, the authors prefer another definition of an ovarian cyst: a persistent dominant follicle with a diameter of ≥ 10 mm, in the absence of a corpus luteum.
Beyond the definitions and classifications of ovarian cysts, there are other questions that the clinician should ask:
- Are ovarian cysts a pathology in their own right or simply a symptom, a consequence of something else, e.g., a dysfunction in the transition phase?
- From a therapeutic point of view, does it make sense to treat the cyst, or should the gynecological clinician rather worry about impregnating the cow with a cystic degeneration of the ovary?
Classification of ovarian cysts
Ovarian cysts have long been classified as type I or follicular ovarian cysts and type II or luteal ovarian cysts.
|
Type of cyst |
Hormonal Production |
Therapy |
|
Follicular Cyst |
Estrogens. 100 ng/ml estrogen in cystic fluid |
May regress with GnRH treatment |
|
Luteal Cyst |
Progesterone. 100 ng/ml progesterone in cystic fluid |
Regress after treatment with PGF2a |
|
Type III Cyst |
No hormonal production |
No effect of GnRH and/or PGF2a |
Table 1. Classification of ovarian cysts according to hormone production.
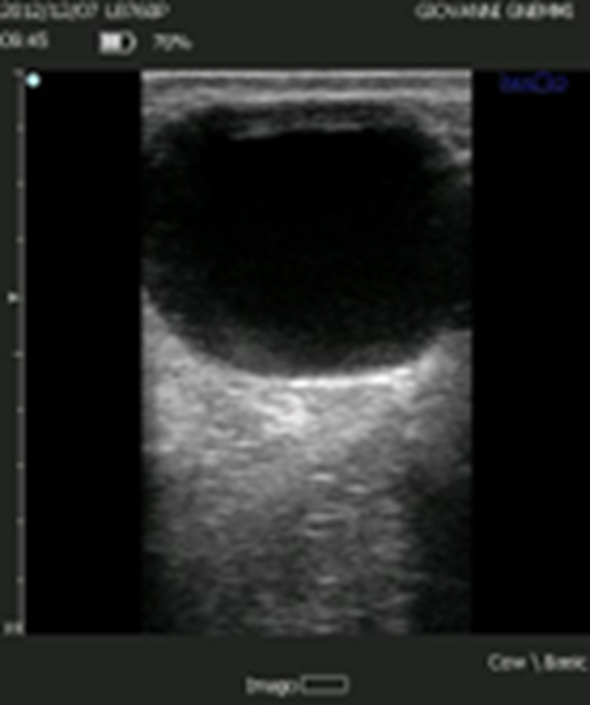
Figure 2. The follicular cyst according to the classical definition presents as a structure of significant diameter (> 20-25 mm), with a thin wall and in the absence of a CL.
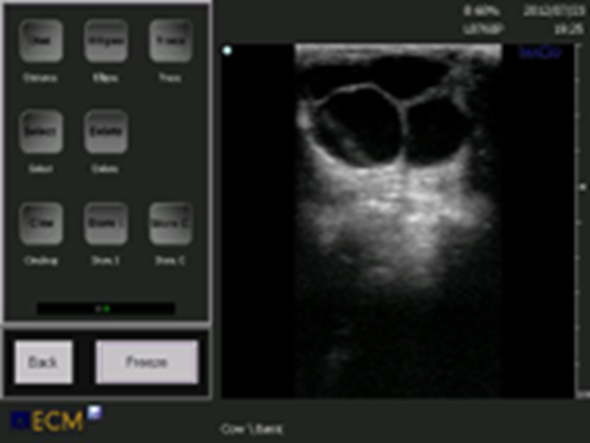
Figure 3. Ovarian cysts may be multiple or single, or separated into different compartments. They may be unilateral or bilateral.
Follicular Cyst
Follicular cysts are usually interpreted by clinicians as large diameter, thin-walled cysts with an estrogen content of ≥ 100 ng/ml in the follicular fluid. They are cystic formations that may regress if treated with GnRH. They may be mono or bilateral; the latter usually have a worse prognosis.
Today, follicular cysts are divided into small and large follicular cysts. Their origin is distinct:
- Small cysts are associated with a state of deep negative energy balance, such that LH pulsatility is blocked. The dominant follicle that has just been deviated in the absence of LH can only regress. After about 24 hours there is a new recruitment and after four days a new deviation and regression of the newly formed dominant follicle. This situation is repeated because of the duration of this negative energy balance.
- Large cysts, on the other hand, arise due to the absence, delay, or insufficiency of the pre-ovulatory peak of LH. When the Graf follicle fails to ovulate, it continues to grow stimulated by the pulsatility of the LH still present, thus giving rise to a large cyst. Interestingly, however, in most cystic cows there does not appear to be a reduced release of GnRH by the hypothalamus, much less a reduction in GnRH receptors in the pituitary, or a reduction in LH content in the pituitary does not appear to be reduced in cystic cows (5, 3). It is therefore assumed that there may be an alteration in the positive feedback of estradiol that controls LH secretion and also in the negative feedback of progesterone, in the absence of which we are in the presence of abnormal pulsatile LH frequencies responsible for the increase in diameter of the pre-ovulatory Graf follicle (3). In the pathogenesis of the follicular cyst, it must also be considered that the level of estrogen leads to an increased production of inhibin, i.e., there is an increased suppression of the FSH dominant follicle, thus establishing a longer lasting dominant phase, during which the dominant follicle increases in diameter. When this cystic follicle goes into regression and no longer produces enough estrogen and inhibin to block FSH, there will be new follicular recruitment due to a peak in FSH. A new dominant follicle then develops, which, if conditions have not changed, will suffer the same fate as the previous dominant follicle, i.e., it will become cystic. The lack of a per-ovulatory LH peak can have different origins:
a) It can be caused by the absence of the estrogenic peak in the Graf follicle. A blockage of aromatase results in the failure to convert androgens into estrogen. This blockage can be caused by an increase in cortisol, e.g., during both Gram + and Gram - mastitis, or even under conditions of heat stress.
b) May depend on hypothalamic desensitization, e.g., in cows with a calf that sucks milk continuously.
c) It may be due to a lack of LH in the adeno-pituitary area, for example in cattle that have lost a lot of weight in the first 3-4 weeks postpartum and/or have suffered from puerperal diseases.
Small follicular cysts are associated with a deeper negative energy balance and are therefore clinically more difficult to resolve than large follicular cysts.
Cows with follicular cysts are often simultaneously or previously exposed to various types of stress, negative energy balance, reduced or poor liver function and a low level of circulating (IGF)-1 (7, 6, 3). An association has been found between these stressors and an increase in Heat Shock Protein (HSP) in ovaries of cows with follicular cysts: altered expression of HSP genes is thought to decrease apoptosis in the follicular wall and lead to delayed regression of cystic follicles (7, 3).
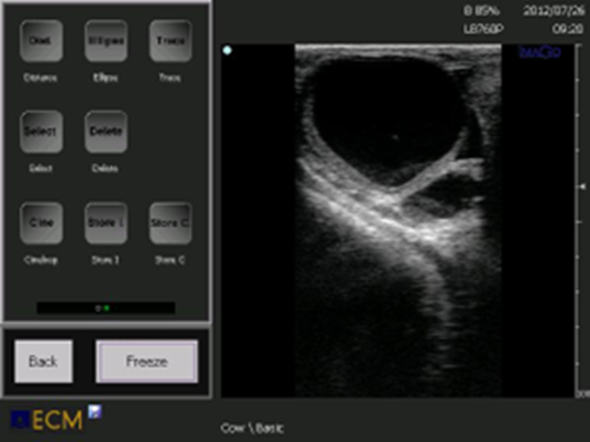
Figure 4. A luteinic cyst is classically defined in comparison to a follicular cyst and has a thicker wall. Most cysts diagnosed as luteinic are actually cavitary corpora lutei or follicular cysts in luteinization.
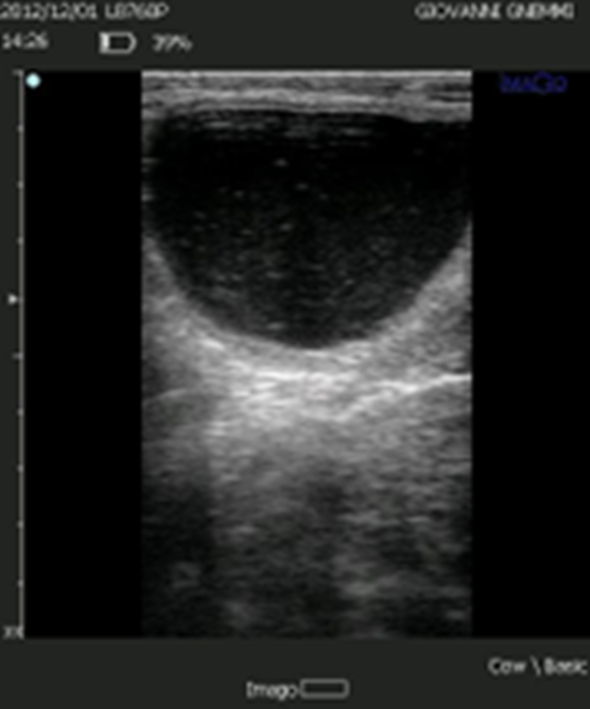
Figure 5. The lutein cyst is now considered to derive from a follicular cyst where luteinization of varying degrees of the wall has been established.
Luteal Cyst
The lutein cyst has a thicker wall (< 3 mm, > 1 mm); it has a progesterone content in the follicular fluid ≥ 100 ng/ml. It regresses if treated with prostaglandin.
Both types of cysts are different forms of the same condition: the luteal cyst is originally a follicular cyst in which the theca and granulosa cells have undergone luteinization and thus have begun to produce progesterone, in a degree directly proportional to the degree of luteinization they have achieved. (5, 3).
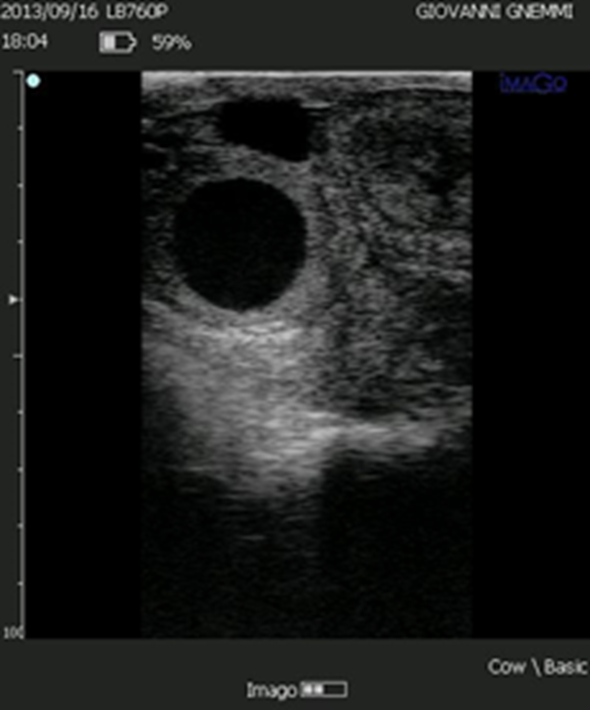
Figure 6. The luteal volume of some structures defined as luteal cysts is such that they produce large amounts of progesterone.
Follicular cysts according to the classification would have thin walls. Even this »certainty« is lost by analyzing ultrasound pictures of ovarian cysts, from which one can easily deduce that even old follicular cysts can have a wall several millimeters thick (2-5 mm). If one wants to make a differential diagnosis between the two types of cysts based on wall characteristics, one should not focus on the thickness but on the echogenicity of the wall, which is different in the two types of cysts. In luteal cysts the wall is hypo-echogenic (grey), whereas in follicular cysts it is connective, i.e., echogenic (white).
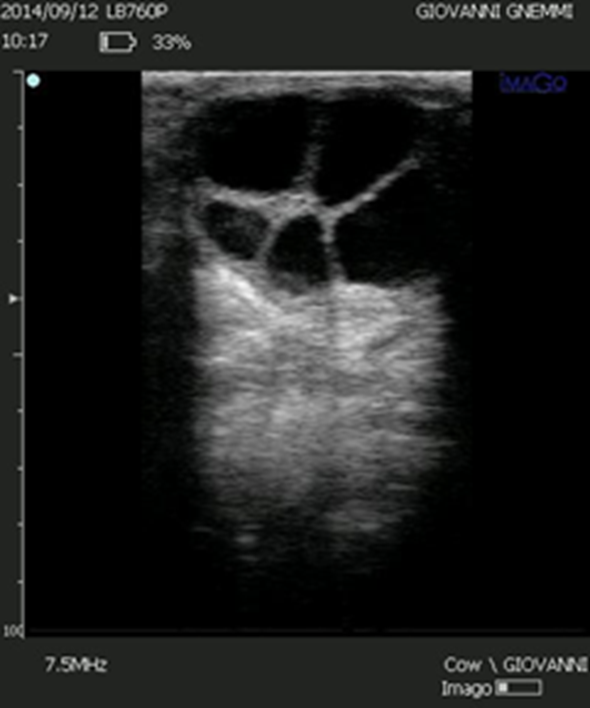
Figure 7. In the differential diagnosis between a follicular cyst and a luteal cyst, the thickness of the wall, which is thinner in the follicular cyst, is always indicated. However, the two ovarian cysts should be differentiated based on the echogenicity of the wall rather than the thickness. This image shows a follicular cyst with a wall thickness of 3-4 mm.
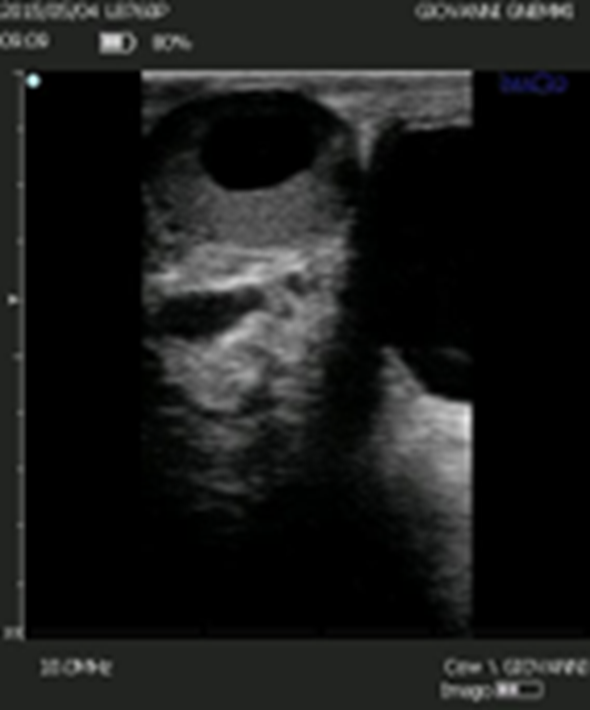
Figure 8. The differential diagnosis between luteal cyst and corpus luteum cavitarium is based on the thickness of the luteal wall. However, it is not possible to use this criterium as the distribution of luteal tissue in the corpus luteum cavitarium is not uniform.
Ovarian cyst Type III
In recent years, cystic structures similar to follicular cysts have been classified as type III ovarian cysts. These structures have a connective and therefore echogenic wall, which is usually very thin. The cystic fluid contains neither estrogen nor progesterone. These structures do not regress when treated with GnRH or PGF2a. If broken or aspirated, they reform. They have been identified in first birth cows. Histologically, no basement membrane, granulosa or theca cells can be identified. They do not respond to any treatment normally employed. The pathogenesis of these cysts is unknown, and the diagnosis is made based on their irreversibility.
Luteal cyst or cavitary corpus luteum?
How many of the luteal cysts that are diagnosed daily are really luteal cysts and how many are cavitary corpora lutei?
If we take the classical definition of a luteal cyst at face value, the wall thickness of the cyst should be < 3 mm and > 1 mm, with a cavity diameter > 5 mm. In the case of the corpus luteum cavitarium the order is reversed: luteal wall thickness > 3 mm, cavity diameter ≤ 5 mm.
In the opinion of the writers, this is an inadequate criterium for differentiating a luteal cyst from CL, given the enormous heterogeneity of cavitary CL: apart from the diameter of the cavity, very often > 5 mm, the distribution of the luteal tissue that surrounds the cavity in the case of cavitary CL is not homogeneous and therefore it becomes impossible to use the above criterium to make a differential diagnosis (8) Today, it is considered that more than 90 % of the structures that are diagnosed as luteal cysts every day are in fact cavitary CL, or follicular cysts in luteinization. But above all, apart from the diagnosis, the therapeutic approach to deal with a cavitary CL, a luteal cyst or a follicular cyst in luteinization is identical and involves the use of a PGF2a prostaglandin.
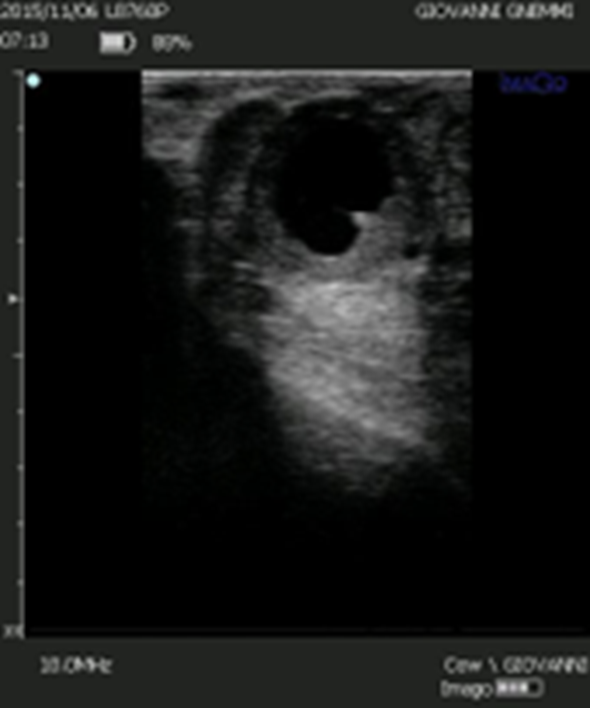
Figure 9. Some cystic structures are difficult to characterize even by ultrasound. However, the presence of luteal tissue in the wall and the presence of luteinization in the cavity should direct the clinician towards luteolytic rather than luteinizing therapy.
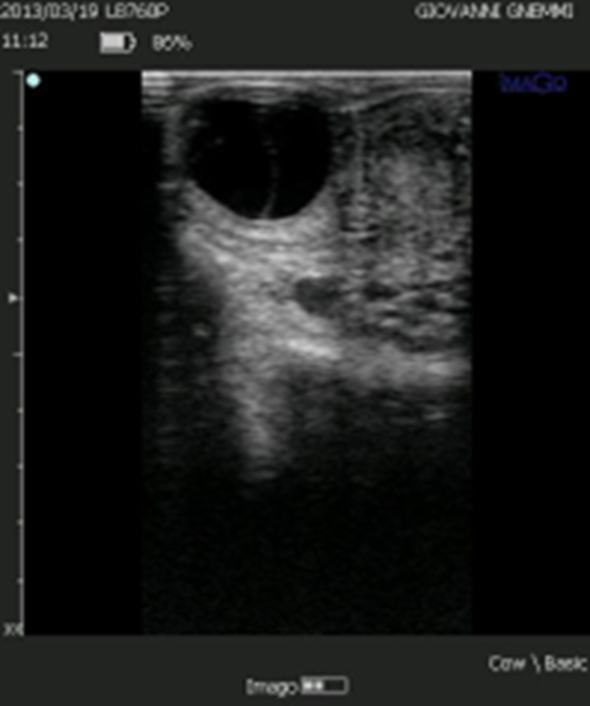
Figure 10. The presence of a luteal structure, surrounded by several large follicular cysts, sometimes puzzles the clinician. However, the presence of luteal tissue should direct the veterinarian towards luteolytic therapy.
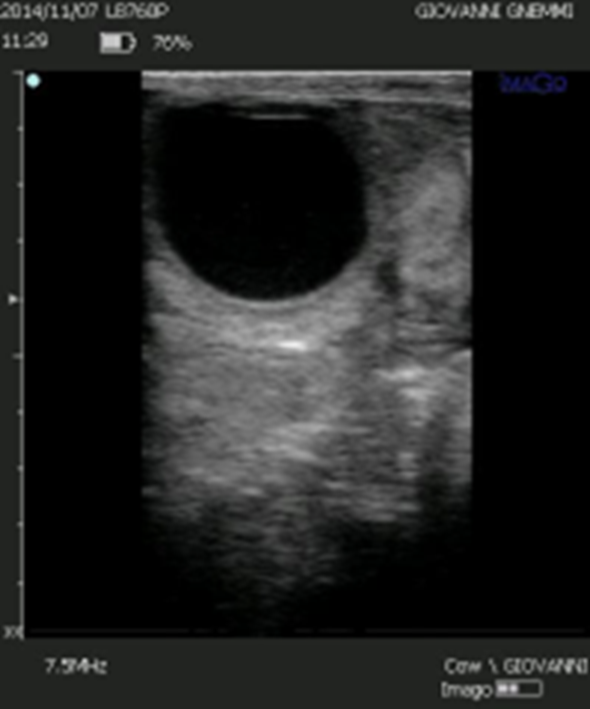
Figure 11. The presence of a large cystic cavity and a thin luteinized wall should not deceive us: If we develop the luteal volume of these structures, we discover a volume of 1.400-1.500 mm3, as much as a compact corpus luteum with a diameter of 20 mm.





